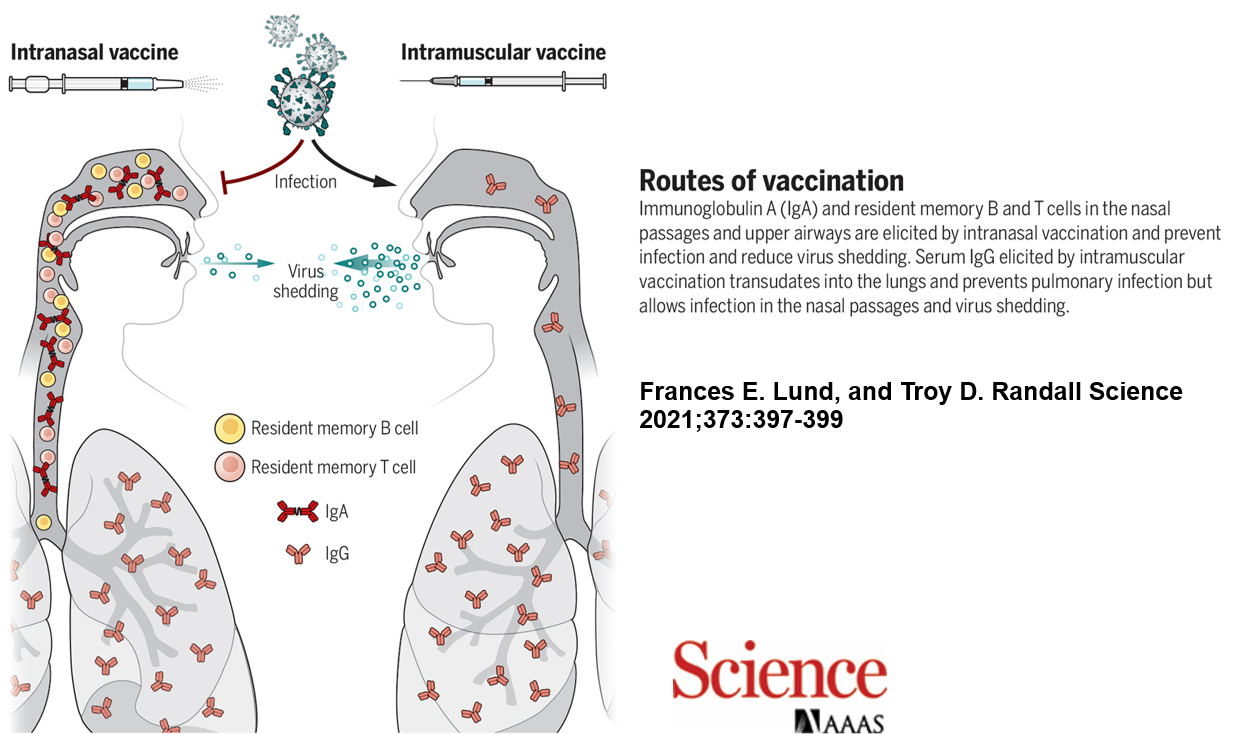
Most infectious diseases begin at mucosal surfaces—especially in the respiratory tract. Unlike traditional intramuscular vaccines, intranasal vaccines create a first line of defense directly at the site of pathogen entry by inducing secretory IgA antibodies. These antibodies not only block infection and reduce transmission but also exhibit broader cross-reactivity against viral variants due to their structural properties.
Beyond superior immunological advantages, intranasal vaccines are needle-free, less invasive, and more cost-effective. They minimize needle-related anxiety, reduce medical waste, and lower the risk of secondary infections. These benefits have positioned intranasal vaccines at the forefront of next-generation immunization strategies. The COVID-19 pandemic further highlighted their importance, driving global recognition and investment in mucosal vaccine development.
Challenges in Intranasal Vaccine Development
The principle of vaccination is straightforward: present the immune system with antigens that safely elicit protective responses without causing disease. Achieving this without injection into blood or muscle, however, remains highly challenging. Developing an intranasal vaccine that is both safe and strongly immunogenic requires solving complex issues in formulation, delivery, and durability of immune protection.
Intranasal vaccines can induce IgA antibodies and tissue-resident memory lymphocytes in the respiratory mucosa, which injectable vaccines cannot achieve.
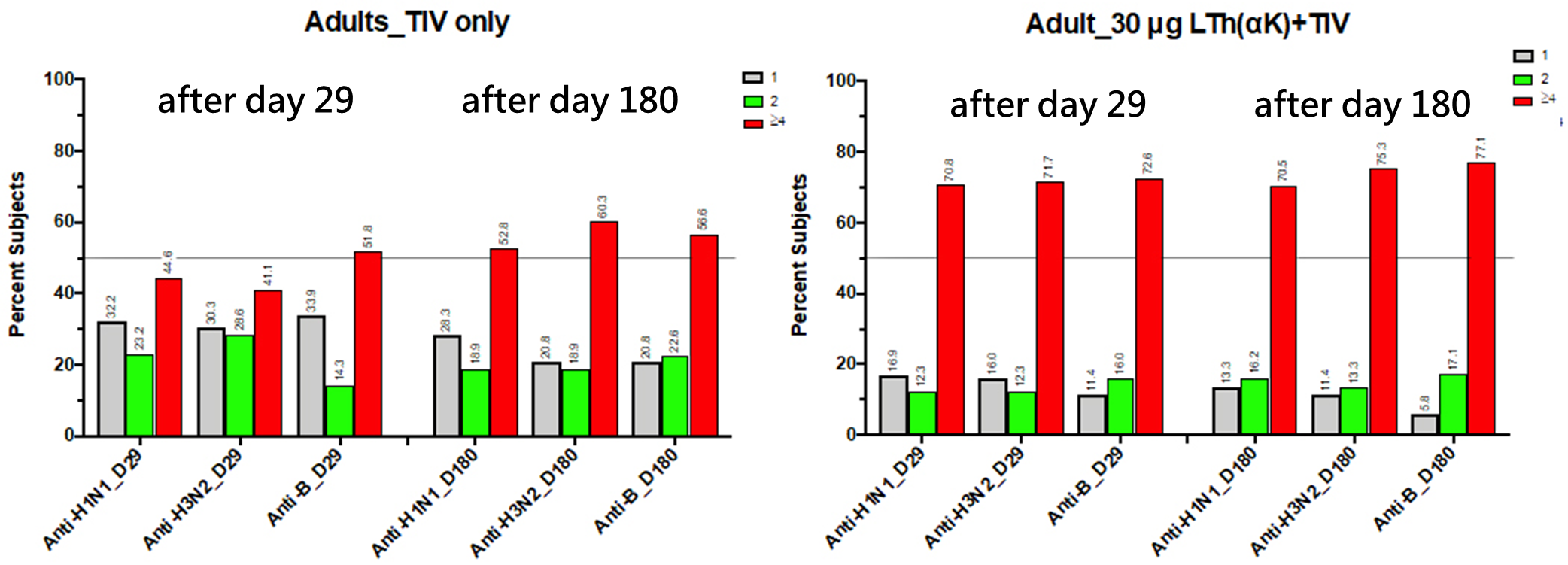
Clinical trials have confirmed that Advagene's intranasal vaccine induces virus-neutralizing mucosal IgA antibodies that persist for over six months.